Trending...
- OneSolution® Expands to Orlando with New Altamonte Springs Implant Center
- Robert DeMaio, Phinge Founder & CEO, Ranked #1 Globally on Crunchbase, Continues to Convert Previous Debt Owed to Him by Phinge into Convertible Notes
- 2025: A Turning Point for Human Rights. CCHR Demands End to Coercive Psychiatry
SHF 2.0 Framework as Practical Solution to Drive Continuous Improvement
MIDDLETOWN, N.Y. - OhioPen -- Ongoing research reveals a notable gap between present practices and the essential best practices needed for successful Human Factors Engineering (HFE) projects. This underlines a significant obstacle within the medical device industry – the ineffective implementation of HFE. The issue has become more apparent in recent years, as the Food and Drug Administration (FDA) now reviews HFE project results as part of their quality system regulation (QSR). Disturbingly, over 90% of HFE submissions are reportedly rejected by the FDA, due to deficiencies.
The Critical Role of Human Factors Engineering (HFE) in the Medical Device Industry
Human Factors Engineering (HFE) has a pivotal role in the medical device industry, serving as the intersection of human capabilities and system operations. It significantly contributes to safety, efficiency, and user experience. However, project failure can result in device designs that jeopardize effective and safe usage.
Pioneering this area of research, Dr. Katia M. Rojas, an expert in project management and operational excellence, has been studying the underlying causes of these project failures. Her groundbreaking research began during her Ph.D. studies in Industrial & Systems Engineering at Binghamton University.
Dr. Rojas' work has unveiled substantial insights into the root causes of failures and the crucial success factors in FDA HFE validation projects. Such observations have led her to assert that HFE validations are currently project managed at relatively low maturity levels, which significantly impacts project quality and success rates. This emphasizes the need for an industry-focused project management maturity framework to facilitate the standardization of emerging best practices.
More on Ohio Pen
"By focusing on improving project management maturity, particularly emphasizing the key success factors or best practices, we can drastically cut down the current 90% rejection rate at the FDA," said Dr. Rojas. "This approach not only addresses the pressing challenges in the medical device industry, but also promises improved project outcomes and enhanced patient safety."
Unveiling a Silent Crisis: Importance of Addressing Findings
In the fast-paced world where innovation and patient safety converge, patients are heavily reliant on the successful application of human factors engineering during medical device development. However, "the unsatisfactory application of HFE has subtly transformed into a silent crisis," Dr. Rojas pointed out.
The alarming FDA rejection rates highlight the urgent need for proactive engagement from key stakeholders to implement corrective measures. Nevertheless, the lack of such engagement raises concerns about the effectiveness of any current interventions aimed at reducing the persistently high failure rate.
Introducing the Successful Human Factors™ 2.0 (SHF 2.0) Framework
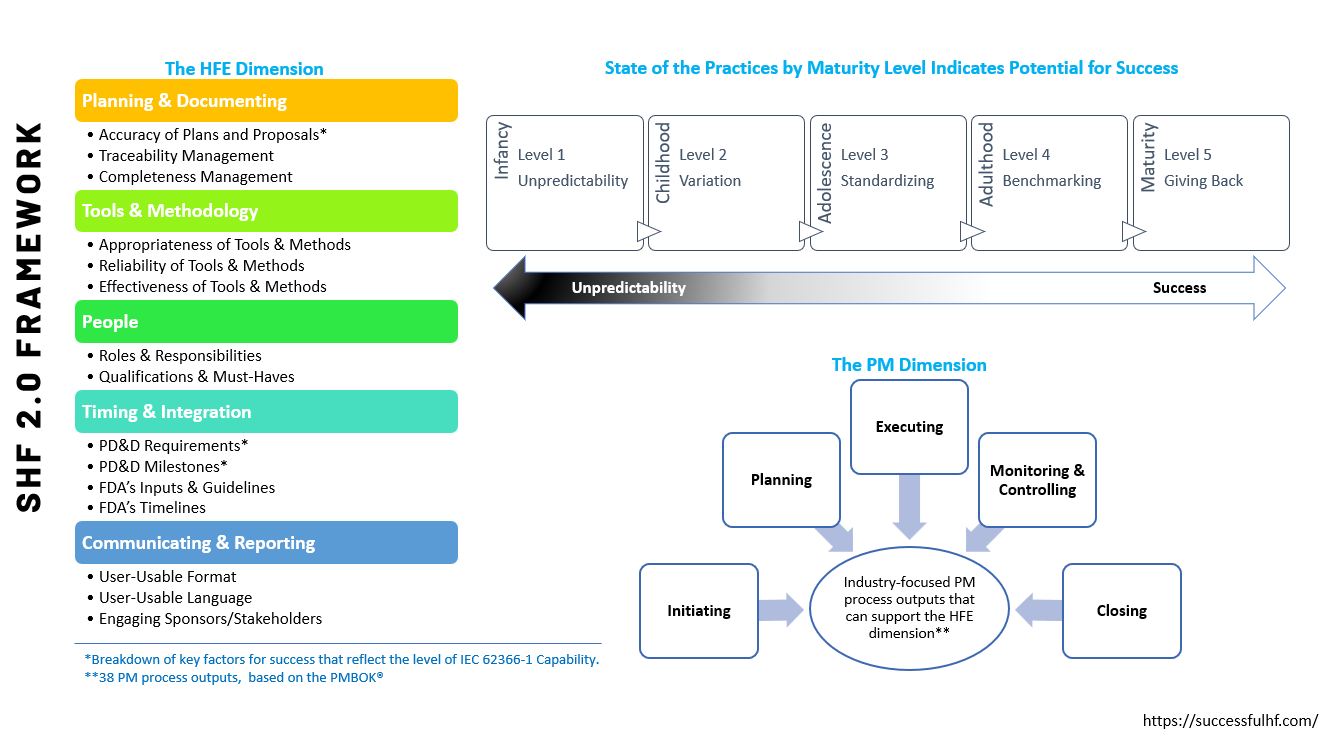
To address these challenges, Dr. Rojas introduces the Successful Human Factors™ 2.0 (SHF 2.0) framework. SHF 2.0 is a researched-based maturity framework designed to optimize HF projects by focusing on key success factors, particularly those related to the delivery of an HFE Validation Report for FDA review. This approach will enable HF service providers (HFSPs) to refine their processes, establish consistent practices, and uphold the highest standards in medical device HFE.
Effectiveness of the SHF 2.0 Framework in Enhancing HF Project Management Maturity
A study investigating the effectiveness of the SHF 2.0 framework found that the average maturity level of successful HF projects corresponded to "Level 2 – Childhood" in the framework, revealing significant variations and a lack of standardization in implementing HFE best practices for medical devices. These findings underline the urgent need for a more standardized and consistent approach in the field.
More on Ohio Pen
Initial investigations validating the SHF 2.0 framework have demonstrated its potential and reliability as a tool for improving the maturity of HF project management. Participants of the study recognized its potential to enhance the success rates of HF submissions.
These key findings have been consolidated in a pre-print publication titled "Validating a Project Management Maturity Framework Based on Emerging Best Practices for Successful Human Factors Projects Requiring FDA Approval – Summary of Key Findings," which is available on ResearchGate.
Future Outlook: Ongoing Research and Embracing the Long Road Ahead
"While the urgent need for improved HF project management practices in the medical device industry is apparent, stakeholders may still be in the early stages of understanding the value of the SHF 2.0 framework," Dr. Rojas noted, acknowledging the long journey ahead. "Broader adoption of the SHF 2.0 framework can significantly improve HF project outcomes, contributing to progress in HFE within the medical device industry."
The SHF 2.0 framework is designed for continuous improvement, encouraging HFSPs to benchmark their performance against industry standards and best practices. This drive towards excellence fosters innovation and creates new industry benchmarks.
To learn more about SHF 2.0, participate in ongoing industry-wide benchmark research, or explore the current industry maturity level report, visit: https://www.successfulhf.com/
The Critical Role of Human Factors Engineering (HFE) in the Medical Device Industry
Human Factors Engineering (HFE) has a pivotal role in the medical device industry, serving as the intersection of human capabilities and system operations. It significantly contributes to safety, efficiency, and user experience. However, project failure can result in device designs that jeopardize effective and safe usage.
Pioneering this area of research, Dr. Katia M. Rojas, an expert in project management and operational excellence, has been studying the underlying causes of these project failures. Her groundbreaking research began during her Ph.D. studies in Industrial & Systems Engineering at Binghamton University.
Dr. Rojas' work has unveiled substantial insights into the root causes of failures and the crucial success factors in FDA HFE validation projects. Such observations have led her to assert that HFE validations are currently project managed at relatively low maturity levels, which significantly impacts project quality and success rates. This emphasizes the need for an industry-focused project management maturity framework to facilitate the standardization of emerging best practices.
More on Ohio Pen
- Lipps Electric Celebrates Nearly Four Decades as a Trusted, Family-Owned Electrical Services Company
- FrostSkin Launches Kickstarter Campaign for Patent-Pending Instant-Chill Water Purification Bottle
- The New Monaco of the South (of Italy)
- Floor2Future Launches to Strengthen the Manufacturing Workforce
- Lkpfm corporation Happy New Years announcement
"By focusing on improving project management maturity, particularly emphasizing the key success factors or best practices, we can drastically cut down the current 90% rejection rate at the FDA," said Dr. Rojas. "This approach not only addresses the pressing challenges in the medical device industry, but also promises improved project outcomes and enhanced patient safety."
Unveiling a Silent Crisis: Importance of Addressing Findings
In the fast-paced world where innovation and patient safety converge, patients are heavily reliant on the successful application of human factors engineering during medical device development. However, "the unsatisfactory application of HFE has subtly transformed into a silent crisis," Dr. Rojas pointed out.
The alarming FDA rejection rates highlight the urgent need for proactive engagement from key stakeholders to implement corrective measures. Nevertheless, the lack of such engagement raises concerns about the effectiveness of any current interventions aimed at reducing the persistently high failure rate.
Introducing the Successful Human Factors™ 2.0 (SHF 2.0) Framework
To address these challenges, Dr. Rojas introduces the Successful Human Factors™ 2.0 (SHF 2.0) framework. SHF 2.0 is a researched-based maturity framework designed to optimize HF projects by focusing on key success factors, particularly those related to the delivery of an HFE Validation Report for FDA review. This approach will enable HF service providers (HFSPs) to refine their processes, establish consistent practices, and uphold the highest standards in medical device HFE.
Effectiveness of the SHF 2.0 Framework in Enhancing HF Project Management Maturity
A study investigating the effectiveness of the SHF 2.0 framework found that the average maturity level of successful HF projects corresponded to "Level 2 – Childhood" in the framework, revealing significant variations and a lack of standardization in implementing HFE best practices for medical devices. These findings underline the urgent need for a more standardized and consistent approach in the field.
More on Ohio Pen
- Lick Personal Oils Introduces the Ultimate Valentine's Day Gift Collection for Romantic, Thoughtful Gifting
- The Best IP Protection for Solo Inventors and Tinkerers Using Instant IP
- Copyright vs. Patent: What Inventors Need to Know
- Tom Quigley of ClaimLinx Warns Americans as Enhanced ACA Subsidies Officially Expire
- Lacy Hendricks Earns Prestigious MPM® Designation from NARPM®
Initial investigations validating the SHF 2.0 framework have demonstrated its potential and reliability as a tool for improving the maturity of HF project management. Participants of the study recognized its potential to enhance the success rates of HF submissions.
These key findings have been consolidated in a pre-print publication titled "Validating a Project Management Maturity Framework Based on Emerging Best Practices for Successful Human Factors Projects Requiring FDA Approval – Summary of Key Findings," which is available on ResearchGate.
Future Outlook: Ongoing Research and Embracing the Long Road Ahead
"While the urgent need for improved HF project management practices in the medical device industry is apparent, stakeholders may still be in the early stages of understanding the value of the SHF 2.0 framework," Dr. Rojas noted, acknowledging the long journey ahead. "Broader adoption of the SHF 2.0 framework can significantly improve HF project outcomes, contributing to progress in HFE within the medical device industry."
The SHF 2.0 framework is designed for continuous improvement, encouraging HFSPs to benchmark their performance against industry standards and best practices. This drive towards excellence fosters innovation and creates new industry benchmarks.
To learn more about SHF 2.0, participate in ongoing industry-wide benchmark research, or explore the current industry maturity level report, visit: https://www.successfulhf.com/
Source: Successful Human Factors™
0 Comments
Latest on Ohio Pen
- Phinge Founder & CEO Robert DeMaio Ranked #1 Globally on Crunchbase, Continues to Convert Previous Debt Owed to Him by Phinge into Convertible Notes
- Donna Cardellino Manager/Facilitator Signs Justin Jeansonne Country Singer-Songwriter To Exclusive Management Deal For Global Music Expansion
- Golden Paper Launches a New Chapter in Its Americas Strategy- EXPOPRINT Latin America 2026 in Brazil
- UK Financial Ltd Executes Compliance Tasks Ahead Of First-Ever ERC-3643 Exchange-Traded Token, SMCAT & Sets Date For Online Investor Governance Vote
- TheOneLofi2: New Home for Chill Lo-Fi Hip Hop Beats Launches on YouTube
- eJoule Inc Participates in Silicon Dragon CES 2026
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
- Kaltra Launches Next-Gen MCHEdesign With Full Integration Into MCHEselect — Instant Simulation & Seamless Microchannel Coil Workflow
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
- Guests Can Save 25 Percent Off Last Minute Bookings at KeysCaribbean's Village at Hawks Cay Villas
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- Insivia Named Sole Recipient of 'Top Strategic Consultancy and Marketing Agency 2026' Award
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- Healthcare Executive Derek Streich Launches Professional Website with Derek Streich Video Biography
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Leimert Park Announces Weeklong Kwanzaa Festival & Kwanzaa Parade Celebrating Black History, Culture, and Community
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book