Trending...
- Peak 10 Marketing Expands Capabilities and Opens Doors to New Clients
- How LIB's Temperature & Humidity Chamber & Walk-in Chamber Warranty Delivered Real Uptime
- Aktion Associates Acquires Deltek Practice from AMR Group
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Continues Expansion with Completion of Dura Medical Acquisition in Network of Interventional Psychiatry Clinics
MIAMI - OhioPen -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression With Suicidality; Assuming Coverage with Buy and $40 Price Target.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
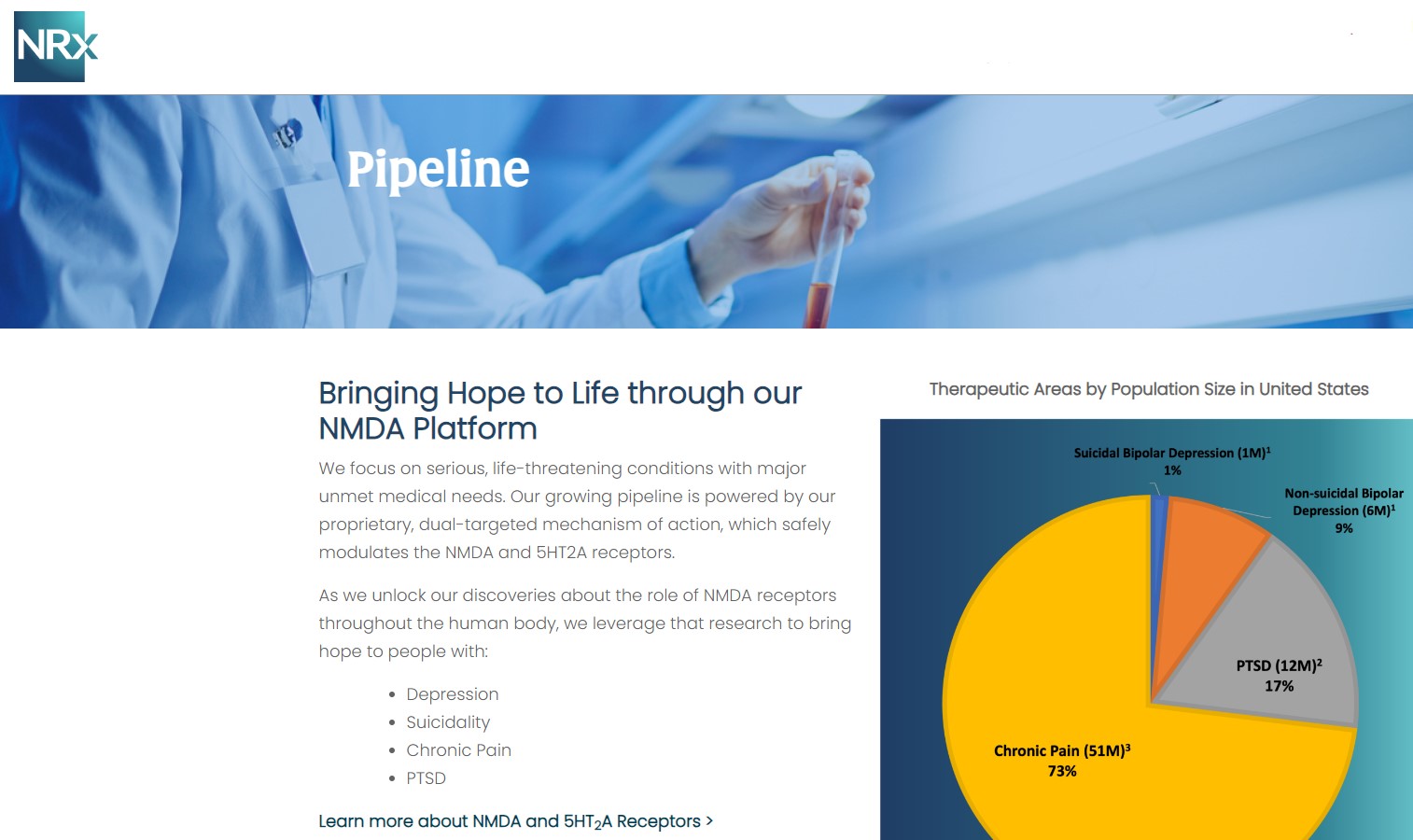
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
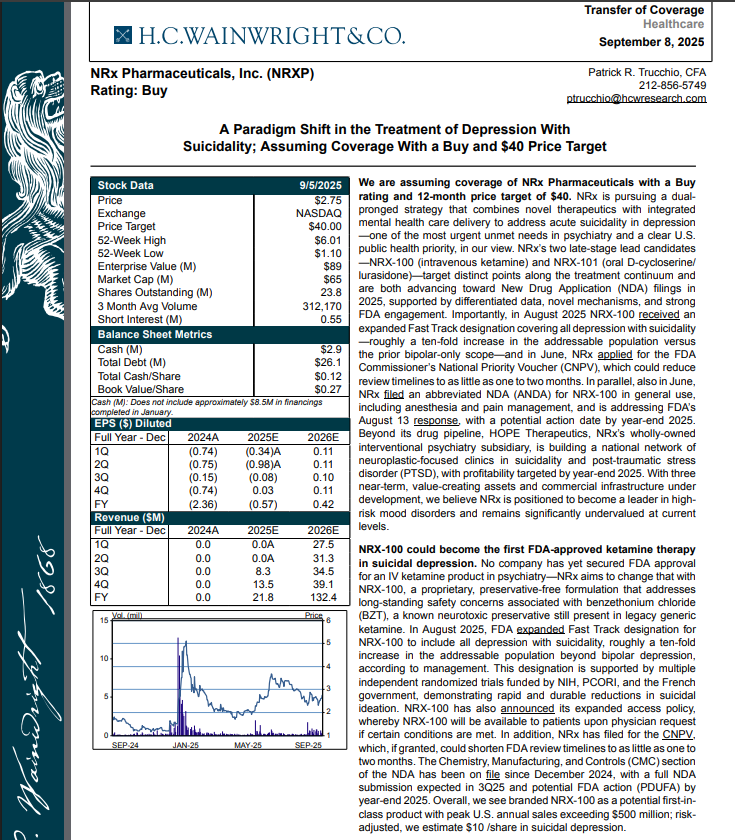
On September 8th H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on Ohio Pen
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Expanded Access Policy for NRX-100 (preservative-free ketamine)
On August 27th NRXP announced its expanded access policy for NRX-100 (preservative-free ketamine) based on grant of Fast Track designation for NRX-100 in the treatment of suicidal ideation in patients with depression, including bipolar depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet need, based on an assessment of the preliminary data contained in the Fast Track designation request. Accordingly, NRXP NRX-100 is available for expanded access to eligible patients.
Second Quarter 2025 Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
More on Ohio Pen
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Completion of a toxicology assessment of Benzethonium Chloride1, documenting its lack of "Generally Recognized as Safe" (GRAS) status and lack of safety data to support its use in intravenous presentations of ketamine.
NRXP filing of a Citizen's Petition with the U.S. Food and Drug Administration to seek the removal of benzethonium chloride, a toxic preservative, from all ketamine products for intravenous administration.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
HOPE Therapeutics
NRXP execution of definitive Purchase Agreement and receipt of final regulatory clearance from Florida's Agency for Health Care Administration ("ACHA") to proceed with closing the acquisition of Dura Medical.
Execution of binding letter of intent to acquire the assets of NeuroSpa TMS Holdings of Tampa, FL.
Execution of a binding letter of intent to acquire a 49% interest in Cohen and Associates, LLC.
NRXP Receipt of approval, pending legal stipulations, for $7.8 million of debt financing to support the acquisition of Dura Medical, NeuroSpa TMS Holdings, and Cohen and Associates, LLC.
Corporate (subsequent to the filing of form 10-Q)
NRXP $6.5 million dollar investment to purchase approximately 3.9 million shares of common stock of NRx Pharmaceuticals on August 18, 2025, by a consortium of experienced biotechnology investors led by B Group Capital. The purchase is subject to a one-year lockup on trading, shorting, or otherwise hypothecating said securities. The investment has no warrants, repricing provisions, commissions, or other structure.
The B Group Capital led consortium of ultra long-term healthcare specialist investors is highly strategic with extensive experience in complex clinical, regulatory, and commercial therapeutics but also direct ownership and management of multi-unit retail operations with potentially positive long-term implications for efforts to continue to scale and develop NRXP HOPE Therapeutics.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression With Suicidality; Assuming Coverage with Buy and $40 Price Target.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
On September 8th H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on Ohio Pen
- Youth Take the Lead: Kopp Foundation for Diabetes Hosts "By Youth, For Youth, With T1D" Gala on October 8 at Blue Bell Country Club
- Green Office Partner Named #1 Best Place to Work in Chicago by Crain's for 2025
- CCHR, a Mental Health Watchdog Organization, Hosts Weekly Events Educating Citizens on Important Mental Health Issues
- "Leading From Day One: The Essential Guide for New Supervisors" Draws from 25+ Years of International Management Experience
- Actor, Musician and Poker Player Nicholas Rascona to Take to ACR Poker Tables to Break Guinness World Record for Longest Marathon Playing Online Poker
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Expanded Access Policy for NRX-100 (preservative-free ketamine)
On August 27th NRXP announced its expanded access policy for NRX-100 (preservative-free ketamine) based on grant of Fast Track designation for NRX-100 in the treatment of suicidal ideation in patients with depression, including bipolar depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet need, based on an assessment of the preliminary data contained in the Fast Track designation request. Accordingly, NRXP NRX-100 is available for expanded access to eligible patients.
Second Quarter 2025 Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
More on Ohio Pen
- New Slotozilla Project Explores What Happens When the World Goes Silent
- The Two Faces of Charles D. Braun: How the Novel, Posthumously Yours, Came to Life
- "The Omniscient Buyer" Launches, Offering a Wake-Up Call for the AI Era of Sales and Marketing
- Counseling Center of New Smyrna Beach Expands Affordable Mental Health Services for Volusia County
- Rise & Rally:Her Legacy Invitational
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Completion of a toxicology assessment of Benzethonium Chloride1, documenting its lack of "Generally Recognized as Safe" (GRAS) status and lack of safety data to support its use in intravenous presentations of ketamine.
NRXP filing of a Citizen's Petition with the U.S. Food and Drug Administration to seek the removal of benzethonium chloride, a toxic preservative, from all ketamine products for intravenous administration.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
HOPE Therapeutics
NRXP execution of definitive Purchase Agreement and receipt of final regulatory clearance from Florida's Agency for Health Care Administration ("ACHA") to proceed with closing the acquisition of Dura Medical.
Execution of binding letter of intent to acquire the assets of NeuroSpa TMS Holdings of Tampa, FL.
Execution of a binding letter of intent to acquire a 49% interest in Cohen and Associates, LLC.
NRXP Receipt of approval, pending legal stipulations, for $7.8 million of debt financing to support the acquisition of Dura Medical, NeuroSpa TMS Holdings, and Cohen and Associates, LLC.
Corporate (subsequent to the filing of form 10-Q)
NRXP $6.5 million dollar investment to purchase approximately 3.9 million shares of common stock of NRx Pharmaceuticals on August 18, 2025, by a consortium of experienced biotechnology investors led by B Group Capital. The purchase is subject to a one-year lockup on trading, shorting, or otherwise hypothecating said securities. The investment has no warrants, repricing provisions, commissions, or other structure.
The B Group Capital led consortium of ultra long-term healthcare specialist investors is highly strategic with extensive experience in complex clinical, regulatory, and commercial therapeutics but also direct ownership and management of multi-unit retail operations with potentially positive long-term implications for efforts to continue to scale and develop NRXP HOPE Therapeutics.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on Ohio Pen
- Crimson Cup Congratulates 15 Independent Coffee Shops in 8 States Marking September Anniversaries
- CCHR: For Prevention, Families Deserve Truth From NIH Study on Psychiatric Drugs
- Sheets.Market Brings Professional Financial Model Templates to Entrepreneurs and Startups
- Webinar Announcement: Investing in the European Defense Sector—How the New Era of Uncertainty Is Redefining Investment Strategies
- AEVIGRA (AEIA) Analysis Reveals $350 Billion Counterfeit Market Driving Luxury Sector Toward Blockchain Authentication
- Galaxy Service Partners Announces Partnership with Thomas Door
- Her Magic Mushroom Memoir Launches as a Binge-Worthy Novel-to-Podcast Experience
- Century Fasteners de Mexico Hires Saúl Pedraza Gómez as Regional Sales Manager in Mexico
- Georgia Misses the Mark Again on Sports Betting, While Offshore Sites Cash In
- VidCliq Launches Faceless2Famous Project with AI Artist Truf as Podcast Host
- Nashville International Chopin Piano Competition Partners with Crimson Global Academy to Support Excellence in Education
- AHRFD Releases Market Analysis: Cryptocurrency Market's Institutional Transformation Accelerating
- Wellness Coach Zakiyyah Broadnax Joins the Dear Black Woman Anthology
- Summit Named to Columbus Business First's Fast 50 for the Fourth Time
- Ubleu Crypto Group Analyzes European Digital Asset Market Opportunities Amid Regulatory Evolution
- NIUFO Examines European MiCA Regulation's Impact on Digital Asset Trading Markets
- Wzzph Analyzes Crypto Market Maturation as Institutional Capital Drives $50B ETF Inflows
- GXCYPX Analyzes South America's Emerging Digital Asset Market Dynamics
- Keyanb Crypto Exchange Positions for Latin America's $600 Billion Remittance Opportunity Amid Global Regulatory Shifts